

TORCH infections are a group of congenital infection and perinatal infection caused by pathogens, toxoplasma gondii (TOX), other pathogens,rubella virus (RV), cytomegalovirus, cytomegalovirus (CMV), herpes simplex virus(HSV) etc., which are passed from mother to child during pregnancy, during delivery, or after birth.
TORCH pathogens can be transmitted vertically through the placenta, causing premature birth, miscarriage, stillbirth or fetal malformation. And through the birth canal, resulting in neonatal multi-system, multi-organ damage and mental retardation of the baby[1]. TORCH screening before and during pregnancy has always been an important project in perinatal health care. Currently, many countries and regions have included TORCH screening in pregnancy screening and issued relevant guidelines[2-8].
TORCH screening measures the immunoglobulin IgM and IgG produced by pathogens in the body to diagnosis infection. Prepregnancy TORCH screening is particularly important to detect acute infection in time, to determine the safe time of pregnancy, to avoid pregnancy during acute infection and active infection, and to provide a basis for interpretation of TORCH screening results during pregnancy.
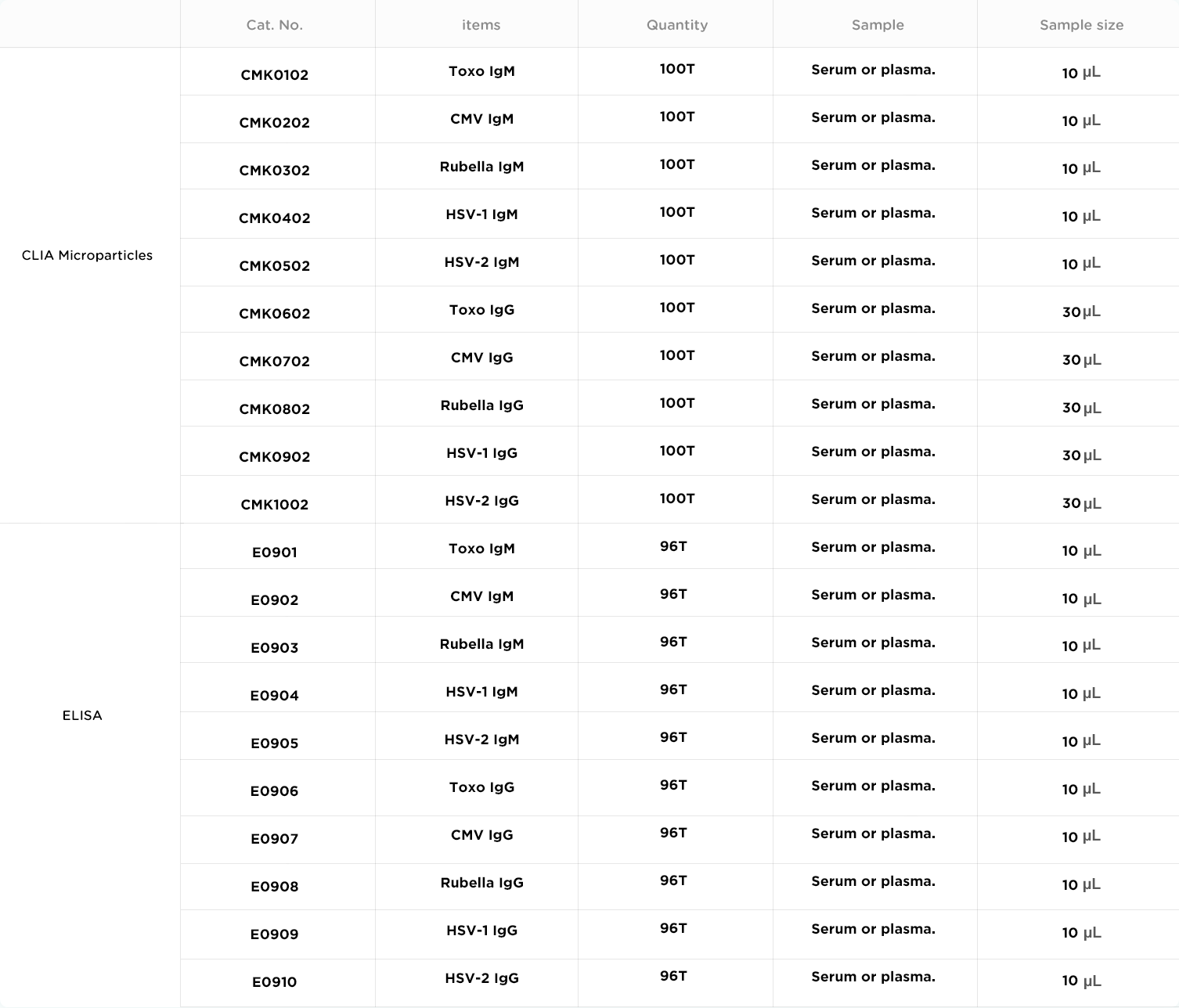
*Autobio is committed to improving the quality of products and services. The specifications and parameters of products are subject to the product manual.
TORCH IgM antibody generally appears 1 week after infection and gradually disappears in 4-8 weeks, which is an indicator of recent infection. IgG antibodies typically appear two weeks after infection and persist for many years, an indicator of previous infection.[9]
Toxo Infection of pregnant women may cause serious consequences to the fetus. Generally, the infection rate of the fetus is low in the first three months of pregnancy (about 17%), and the common symptom is miscarriage. Infection at 4 to 6 months may cause moderate or severe damage to the fetus; The infection rate was higher in late pregnancy (64%), but the fetus had higher resistance and less damage.
CMV is one kind of double-stranded DNA virus. Pregnant women of CMV primary or recurrent infections can cause neonatal intrauterine or perinatal infection, but the harm of primary infection is more serious to fetus than that of recurrence infection. Most congenital infected fetals shows symptom of mental retardation, visually impaired, hearing loss, especially giant cell inclusion body disease (CI, characterized by reticuloendothelial system and invaded the central nervous system), which will cause multiple systems, multiple organ damage to baby.
Rubella is one kind of RNA viruses. During the first 6 months of pregnancy, if pregnant women are infected with Rubella virus, the virus can invade the fetus through the placenta, causing miscarriage and stillbirth, about 29% of the birth baby have "Congenital Rubella Syndrome" (CRS), birth weight less than 2.5 kg, stunted growth; with systemic organ damage, congenital heart disease, deformity, deafness, blindness and so on.
HSV has two serotypes, namely herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2). The transmission routes of the two virus are different: HSV-1 is mainly transmitted by close contact of respiratory tract, skin and mucosa; HSV-2 is transmitted primarily through sex. If the women was primary infected in the first 3-6 months of pregnancy, it will cause congenital infection of the fetus, or abortion, stillbirth, but the probability is very low. If primary infections happens in the last three months of pregnancy, the probability to infect the fetus is about 30-50%.
[1] Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol. 2015 Mar; 42 (1) : 77-103
[2] Yinon Y, Farine D, Yudin MH, et al. Cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can, 2010, 32(4): 348-354.
[3] Dontigny L, Arsenault MY, Martel MJ, et al. Rubella in pregnancy. J Obstet Gynaecol Can, 2008, 30(2): 152-158.
[4] Paquet C, Yudin MH, Society of Obstetricians and Gynaecologists of Canada. Toxoplasmosis in pregnancy: prevention, screening, and treatment. J Obstet Gynaecol Can, 2013, 35(1): 78-81.
[5] Money D, Steben M, Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines:Guidelines for the management of herpes simplex virus in pregnancy. Number 208, June 2008. Int J Gynaecol Obstet, 2009, 104: 167-171.
[6] Patel R, Kennedy OJ, Clarke E, et al. 2017 European guidelines for the management of genital herpe. Int J STD AIDS, 2017, 28(14):1366-1379.
[7] Money DM, Steben M. No. 208-Guidelines for the management of herpes simplex virus in pregnancy. J Obstet Gynaecol Can, 2017, 39(8): e199-205.
[8] Crane J, Mundle W, Boucoiran I, et al. Parvovirus B19 Infection in Pregnancy. J Obstet Gynaecol Can, 2014, 36(12): 1107-1116.
[9] Meek B, Diepersloot RJ, van Gool T, Speijer D,Peek R. Igm recognition of recombinant Toxoplasma gondii antigens by sera of acutely or latently infected humans. Diagn Microbiol Infect Dis. 2003 Jan;45 (1) : 45 to 52